Fracture Risk in Thoroughbred Racehorses: New Genetic Evidence Every Equine Veterinarian Should Know
Fracture risk in Thoroughbred racehorses remains one of the most devastating and emotionally challenging problems in equine practice.
Despite advances in training protocols, track design, and veterinary oversight, catastrophic fractures in racehorses continue to result in approximately 60 equine deaths each year on UK racecourses alone – with global estimates likely exceeding 600–800 annually.
For equine veterinarians, these injuries raise difficult questions: Why do some horses fracture despite optimal management? Can fracture risk be identified earlier? And is fracture susceptibility partly genetic?
A recent study from the Royal Veterinary College (RVC) provides new evidence that genetic fracture risk in horses is measurable at a cellular level, offering insight into why some Thoroughbreds may be biologically predisposed to catastrophic injury – even before clinical signs appear.

Is Fracture Risk in Thoroughbred Racehorses Genetic?
Yes. Recent research from the Royal Veterinary College shows that fracture risk in Thoroughbred racehorses has a measurable genetic component, reflected in distinct gene expression patterns in bone-forming cells (osteoblasts) from high-risk horses.
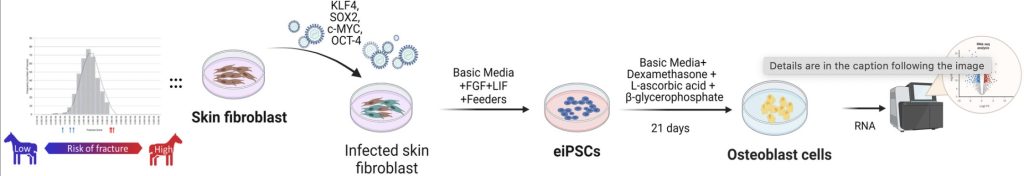
Led by Dr. Debbie Guest, a Senior Research Fellow at the RVC, this study utilized induced pluripotent stem cells (iPSCs) from Thoroughbreds at high and low genetic risk of fracture, as determined by a validated polygenic risk score (PRS).
These stem cells were differentiated into osteoblasts, and their gene expression profiles were analyzed using RNA sequencing.
The Key Findings:
112 genes were differentially expressed between high-risk and low-risk horses.
43 of these genes have known roles in bone biology.
Many of the differentially expressed genes are involved in:
- Extracellular matrix regulation
- Bone remodeling
- Calcium signaling
- Glucose metabolism
Horses with high genetic risk showed decreased expression in key glycolysis pathways, potentially impairing osteoblast function and energy metabolism.
This is the first comprehensive transcriptomic analysis of fracture risk using iPSC-derived osteoblasts in horses, building on previous work that identified COL3A1 (collagen type III) as a key risk-related gene.
Clinical Take-Home Summary
- Fracture risk in Thoroughbred racehorses is influenced by genetic factors, not just training or surface conditions
- Horses at higher genetic risk show altered bone cell metabolism and extracellular matrix regulation
- Genetic risk does not replace mechanical or management factors, but may explain why some horses fracture despite optimal care
- Future screening tools may allow earlier identification of high-risk horses
What This Means Clinically: Actionable Tips for Equine Practitioners
These findings align with clinical observations long recognised by equine veterinarians working in racehorse practice: that some horses sustain fractures despite careful training, optimal nutrition, and appropriate imaging surveillance.
- Genetic Predisposition Is Real (and Measurable)
This study supports what many of us have suspected in practice: some horses simply have “weaker” bones despite optimal management. For breeders and trainers, integrating genetic risk scoring into selection decisions could one day be as routine as scope exams or radiographs.
Clinical Tip: Begin a dialogue with owners and breeders about emerging genetic screening tools – especially for high-value Thoroughbreds or those with a family history of fracture.
- Focus on Metabolic Health of the Bone
Genes related to glycolysis and glucose metabolism were downregulated in high-risk horses. This suggests that poor cellular energy availability may impair osteoblast function and bone repair.
Clinical Tip: Ensure racehorses and young athletes receive adequate energy sources – not just for performance, but potentially for bone integrity. This reinforces the need for careful diet formulation, particularly in horses recovering from bone injury.
- Rethink the Role of the Extracellular Matrix
Several upregulated genes in high-risk horses (e.g., LAMB3, PLA2G4D, SPARCL1) regulate the extracellular matrix (ECM) – which is critical in bone remodeling and signaling.
Clinical Tip: When managing repetitive strain injuries or stress fractures, consider therapies that support ECM remodeling e.g., controlled loading programs, bisphosphonates (with caution), or nutritional supplements targeting collagen synthesis and mineralization.
- Targeted Surveillance for High-Risk Candidates
While PRS testing is not yet commercially available, horses with a known familial history of fractures or unexplained poor healing responses could be flagged for heightened surveillance.
Practical Recommendations:
- Incorporate routine bone density scanning or scintigraphy during training periods for these candidates.
- Institute longer rest intervals post-racing or high-load events.
- Monitor for early signs of stress remodeling using serial imaging.

Limitations and Cautions
While this research is robust and cutting-edge, several limitations apply:
- Small sample size (6 horses) limits generalizability.
- Validation of the PRS in a larger cohort is still pending.
- Functional roles of many differentially expressed genes remain unknown.
- Environmental and mechanical load factors weren’t accounted for in vitro.
Importantly, while this study identifies associations, it does not yet prove causality. We should therefore view these findings as additive to current risk mitigation – not replacements for sound horsemanship and training protocols.
Why This Research Matters
This is a pivotal step in moving equine fracture management from reactive treatment to proactive prevention.
By understanding the genetic “wiring” that predisposes some horses to catastrophic injury, we can begin to tailor both individualized care plans and broader herd management strategies.
For practitioners, this means:
- Being on the frontlines of translating genetic science into equine welfare.
- Helping owners make informed, data-backed decisions.
- Participating in the future development of screening tools and preventive interventions.
The RVC’s work signals a future where bone health is no longer a mystery of fate, but a measurable, manageable risk factor. As equine veterinarians, our job is to integrate this knowledge into practical, compassionate care that protects both the horse’s health and the integrity of our sport.
For further reading, see: Palomino Lago et al. (2025). “Identification of a global gene expression signature associated with the genetic risk of catastrophic fracture in iPSC‐derived osteoblasts from Thoroughbred horses.” Full paper link: https://doi.org/10.1111/age.13504
Frequently Asked Questions About Equine Fracture Risk
Is fracture risk in racehorses genetic?
Yes. Recent research from the Royal Veterinary College shows that horses with a higher genetic risk of fracture demonstrate distinct gene expression patterns in bone-forming cells, suggesting an inherited component to fracture susceptibility.
Can veterinarians test horses for fracture risk?
Currently, genetic fracture risk testing is not commercially available, but research is advancing rapidly. In the future, polygenic risk scores may complement imaging and clinical assessment.
Why do some Thoroughbreds fracture despite good management?
Fracture risk is multifactorial. Genetics, bone metabolism, training load, surface conditions, and recovery time all interact. Genetic predisposition may explain why some horses fracture despite optimal care.
How can vets reduce fracture risk in high-risk horses?
Targeted surveillance, workload modification, nutritional optimisation, and early imaging of stress remodelling may help reduce catastrophic outcomes.